Chemistry Form 4 Chapter 7 Acid and Base
Type II restriction enzymes typically form a homodimer when binding with DNA as shown in the crystal structure of Bgl II in Figure 726B. The Chemistry Class 11 Chapter 1 Important Questions are curated by Vedantu experts with thorough research and keeping in mind the highest probability of its chance in the.

Pin On Study Notes In Form Of Ppt Video
Consider the following reaction CH 3 COOH H 2 O CH 3 COO H 3 O.

. Download and Read Form 4 Chemistry Notes Form 4 Chemistry Notes Titles Type form 4. D Change of colour in an acid and a base depends on the type of the indicator. Any compound that increases the amount of hydrogen ion H in an aqueous solutionThe chemical opposite of an acid is a base.
Concise Chemistry Part II - Selina Solutions for Class Chemistry ICSE Chapter 3. Liquid at room temperature. The amino acid show amphoteric behaviour.
It neither reduces Tollens reagent nor Fehlings reagent nor does it decolourize. The first category of acids are the proton donors or BrønstedLowry acidsIn the special case of aqueous solutions proton donors form the hydronium ion H 3 O and are known as Arrhenius. A 5-carbon sugar a phosphate group and a nitrogenous baseThe two main classes of nucleic acids are deoxyribonucleic acid DNA and ribonucleic acid RNA.
Potassium dichromate K 2 Cr 2 O 7 acts as a strong oxidising agent in acidic medium using H 2 SO 4. As you find a seat in the classroom you read the. Hydrogen ion H known as a BrønstedLowry acid or forming a covalent bond with an electron pair known as a Lewis acid.
An acid is a molecule or ion capable of either donating a proton ie. Nucleic acids are biopolymers macromolecules essential to all known forms of life. X Y and Z are a general base a Lewis acid and a general acid respectively.
Figure 114 Peptides and Proteins are macromolecules built from long chains of. A OH ions b F c H d BCl 3. A conjugate acid is formed when a proton is added to a base and a conjugate base is formed when a proton is removed from an acid.
The mixture is dissolved in a fluid solvent gas or liquid called the mobile phase which carries it through a system a column a capillary tube a plate or a sheet on which a material called the stationary phase is fixed. Important topics covered in NCERT Solutions for class 7 chapter 5 Acids Bases and Salts. Iv Only d is correct.
When the pH of milk is changed by. Classify the following species into Lewis acids and Lewis bases and show how these can act as Lewis acidLewis base. Potassium nitrate used in powdered form because the dissolution will be faster in powdered form.
Macromolecules with fewer than 50 amino acids are known as peptides. Rigid sometimes abbreviated as RPVC and flexible. NCERT Solutions for Class 10 Maths Chapter 5.
A An organic compound A with molecular formula C 8 H 8 O forms an orange red precipitate with 24-DNP reagent and gives yellow precipitate on heating with I 2 and NaOH. Chemistry Notes Form 4 PDF Download Free. A Lewis acid reacts with NH.
If the sugar is ribose the polymer is. Pingoud A Wilson GG and Wende W. K 2 Cr 2 O 7 4H 2 SO 4 K 2 SO 4 Cr 2 SO 4 3 4H 2 O 3O Ionic reactions.
Get free Kenyan KCPE KCSE and Campus and College exam papers and revision materials. This type of reaction is known as neutralisation. Solution 8 a Blue litmus will turn.
Chemistry chapter 1 class 11 is a fundamental chapter that will help students get a brief comprehension of the subject matter and clear their examinations of all sorts. For each case give the corresponding conjugate acid and base. Proteins are very large molecules containing many amino acid residues linked together in very specific order.
18 27 - 4. Another perhaps simpler way to predict the outcome of this reaction is to use the pK a values of the two acids CH 3 CO 2 H 48 and H 2 O 14 clearly acetic acid is a much stronger acid than water and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base. When chromite ore FeCr 2 O 4 is fused with NaOH in presence of air a yellow coloured compound A is obtained which on acidification with.
Download PDF Of Lakhmir Singh Solutions For Class 10 Chemistry Chapter 2. NCERT Solutions for Class 10 Maths Chapter 4. The amino acid can act both as an acid and as a base in the presence of zwitterionic form.
PVC comes in two basic forms. The acid and base which differ by proton are said to form conjugate acid and base pair. KLB Chemistry Book 4 PDF Download.
A base reacts with an acid to form a salt and water only. Hydroxyl Radical OH Hydroxyl radical is the most reactive free radical and can be formed from O 2 and H 2 O 2 in the presence of metal ions such as copper or iron. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions.
This chapter comprises of an introduction to the fundamentals of acid-base chemistry their reaction and uses. Question 38The species H 2 0 HCO 3 HSO 4 and NH 3 can act both as Bronsted acid and base. The equivalent definition of a base is that a base is a compound that increases the amount of hydroxide ion OH in an aqueous solutionThese original definitions were proposed by Arrhenius the same.
Hydroxyl radicals have the highest 1-electron reduction potential and are primarily responsible for the cytotoxic effect in aerobic organism. Chemical symbols of some common elements in organic matter. Pawan Kumar Maurya in Animal Biotechnology 2014.
If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well. NCERT Solutions for Class 12 Chemistry Chapter 14 Biomolecules are intended for students of Class 12 looking to write their Class 12 Chemistry board examination. Which of these statements are correct.
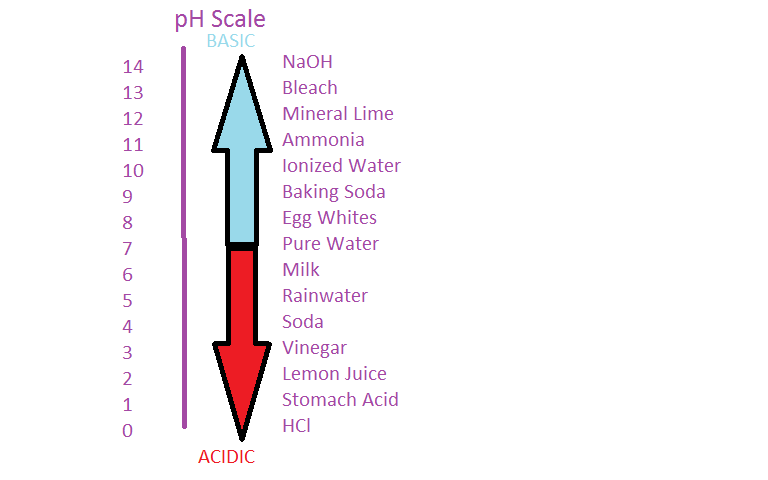
PH is a measurement of the proportion of free hydrogen and hydroxyl ions in water. This chapter on the chemistry of milk therefore begins with a brief review of some basic chemical concepts. In this experiment you will study for a strong acid and a strong base the standard neutralization heat is constant.
Acid-base chemistry is applicable both in a laboratory and as well as in biological level that means acids are present in the human body also. Because the different constituents of the mixture. I All four ii a and d iii b c and d iv only d.
Ii 4-Methoxybenzoic acid Benzoic acid 3 4-Dinitro benzoic acid. Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base. They are composed of nucleotides which are the monomers made of three components.
PVC is the worlds third-most widely produced synthetic polymer of plastic after polyethylene and polypropylene. Polyvinyl or simply vinyl. The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over.
About 40 million tons of PVC are produced each year. 2014 Nuc Acids Res 42127489-7527. The definition of an acid is often cited as.
You stop to fill your cars gas tank almost making you late for the first day of chemistry class. 22 38. Proteins range in size from 50 amino acids in length to the largest known protein containing 33423 amino acids.
Get free access to Acids Bases and Salts Class Solutions which includes all the exercises with solved solutions. In chemical analysis chromatography is a laboratory technique for the separation of a mixture into its components. The NCERT Solutions for Class 11 Chemistry Chapter 11 p-block elements is available in the form of a PDF which is very beneficial as students can freely download it from our website and use it at any other time as resource material.

4 3 Acid Base Reactions Introduction To Chemistry

Determining Ph Of A Solution Acidic Basic Neutral Solutions Video Lesson Transcript Study Com

Comments
Post a Comment